⚠️ Disclaimer: The information provided in this article is for educational purposes only and does not constitute medical advice. RevisionTown does not provide diagnosis, treatment, or medical recommendations. Always consult a qualified healthcare professional regarding any medical condition, symptoms, or concerns.
Read More – 🏥 Medical Disclaimer
Comprehensive Report on Gastroparesis
1. Overview
What is Gastroparesis?
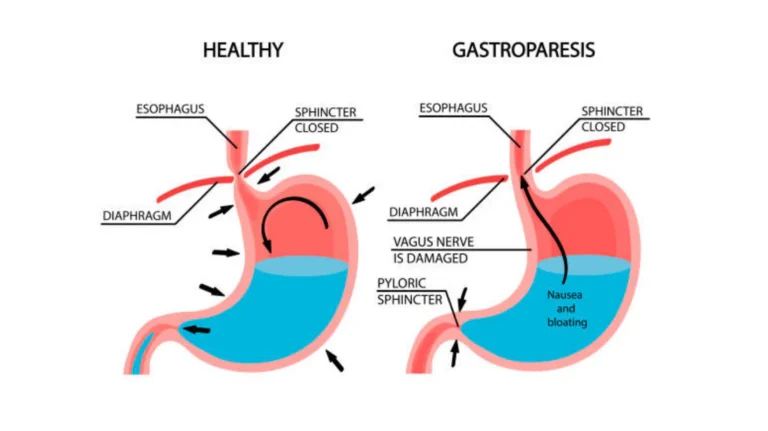
Gastroparesis is a chronic digestive disorder characterized by delayed gastric emptying in the absence of mechanical obstruction. The term “gastroparesis” literally means “stomach paralysis,” reflecting the impaired ability of the stomach to properly contract and propel food into the small intestine. This results in food remaining in the stomach for abnormally long periods, leading to a variety of digestive symptoms and potential complications.
A Concise Yet Detailed Definition
Gastroparesis is a neuromuscular disorder of the upper gastrointestinal tract characterized by delayed gastric emptying without evidence of mechanical obstruction. It involves disruption of the complex coordination between the enteric nervous system, smooth muscle cells, interstitial cells of Cajal (ICC), and the vagus nerve, which normally regulate gastric motility. The result is impaired grinding of food, inadequate mixing with digestive enzymes, and slowed propulsion of stomach contents into the small intestine.
The Affected Body Parts/Organs
While gastroparesis primarily affects the stomach, its impact extends throughout the digestive system and beyond:
Primary Affected Structures:
- Stomach: Particularly the antrum (lower portion responsible for grinding food) and pylorus (the outlet that controls emptying)
- Vagus nerve: The primary nerve controlling gastric motility
- Interstitial cells of Cajal (ICC): Specialized cells that act as pacemakers for gastric contractions
- Gastric smooth muscle: The muscular tissue that executes contractions
- Myenteric plexus: The nerve network within the stomach wall
Secondary Affected Structures:
- Esophagus: Can experience reflux due to increased gastric pressure
- Small intestine: May receive inadequately processed food at irregular intervals
- Pancreas: Altered delivery of nutrients can affect insulin requirements
- Liver: Medication metabolism may be affected by altered gastric emptying
- Colon: Overall GI transit can be affected
Prevalence and Significance of the Disease
Gastroparesis represents a significant healthcare challenge with substantial impacts on patients’ quality of life and healthcare resource utilization:
Prevalence:
- Estimated to affect approximately 1-2% of the general population
- Approximately 4-5 million individuals in the United States
- Up to 40% of patients with type 1 diabetes may have some degree of gastroparesis
- Up to 30% of patients with type 2 diabetes may be affected
- Women are affected approximately four times more frequently than men
Significance:
- Substantial impact on quality of life comparable to end-stage renal disease
- Economic burden estimated at $5-$10 billion annually in the United States
- Up to 30% of patients with severe gastroparesis require frequent hospitalizations
- Average annual healthcare costs for gastroparesis patients can exceed $30,000
- Often underdiagnosed due to symptom overlap with other conditions
- Represents a growing healthcare challenge as diabetes prevalence increases
- Significantly impacts nutritional status, medication effectiveness, and glycemic control
Gastroparesis stands at the intersection of neurological, endocrine, and digestive disorders, making it complex to manage and treat effectively. Its chronic nature and impact on daily activities, nutrition, and overall health underscore the importance of improved understanding, earlier detection, and more effective treatment approaches.
2. History & Discoveries
First Identification
The concept of disordered gastric emptying has ancient roots, but gastroparesis as a distinct clinical entity emerged gradually:
- Early Observations: References to symptoms consistent with gastroparesis appear in medical texts dating back to ancient Egypt and Greece, though not recognized as a specific disorder
- 19th Century: The first systematic descriptions of delayed gastric emptying appeared in medical literature
- 1908: Walter B. Cannon’s pioneering work using X-rays to study digestive motility provided the first visualization of delayed gastric emptying
- 1958: The term “gastroparesis diabeticorum” was first used by Morris Kassander to describe gastric retention in diabetic patients
Key Discoverers
Several key figures contributed to our understanding of gastroparesis:
- Walter B. Cannon (1871-1945): American physiologist who pioneered the use of X-rays to study digestive motility and established fundamental concepts about gastric emptying
- Morris Kassander: First described diabetic gastroparesis as a distinct clinical entity in 1958
- Robert M. Zollinger and Edwin H. Ellison: Their work on gastric secretion and motility in the 1950s contributed to understanding of gastroparesis mechanisms
- W. Grant Thompson: Made significant contributions to understanding non-diabetic gastroparesis in the 1970s
- Kenneth Koch and William Hasler: Modern pioneers in gastroparesis research who developed diagnostic criteria and treatment protocols in the 1990s and 2000s
Major Discoveries and Breakthroughs
The understanding and management of gastroparesis has evolved through several key developments:
Diagnostic Advances:
- 1950s-1960s: Development of radionuclide scintigraphy methods to quantify gastric emptying
- 1976: Introduction of standardized solid-phase gastric emptying studies
- 1992: Development of the first consensus protocol for gastric emptying scintigraphy
- 2000: Validation of the Gastroparesis Cardinal Symptom Index (GCSI) to standardize symptom assessment
- 2008: Establishment of standardized 4-hour gastric emptying protocol by the Society of Nuclear Medicine
Pathophysiological Insights:
- 1980s: Recognition of the role of hyperglycemia in inducing acute gastroparesis
- 1992: Identification of loss of interstitial cells of Cajal (ICC) in diabetic gastroparesis
- 2003: Discovery of the relationship between cellular oxidative stress and gastroparesis
- 2010: Recognition of the role of immune dysregulation and macrophage phenotypes in gastroparesis
- 2013-present: Advances in understanding of the gastric microbiome and its role in motility
Treatment Milestones:
- 1960s: Introduction of metoclopramide, the first prokinetic agent specifically for gastroparesis
- 1980s: Development of domperidone as an alternative prokinetic with fewer central nervous system effects
- 1995: FDA approval of cisapride (later withdrawn due to cardiac concerns)
- 2000: Introduction of gastric electrical stimulation (Enterra therapy)
- 2015: Development of novel gastric per-oral endoscopic pyloromyotomy (G-POEM)
- 2017: Introduction of relamorelin, a novel ghrelin receptor agonist for diabetic gastroparesis
- 2020: FDA approval of prucalopride, offering a new class of prokinetic
Evolution of Medical Understanding
Our understanding of gastroparesis has evolved significantly over time:
1900s-1950s: Mechanical Perspective
- Viewed primarily as a mechanical failure of the stomach
- Limited understanding of neurological factors
- Few effective treatments or diagnostic methods
- Often confused with psychosomatic conditions
1960s-1980s: Diabetic Focus
- Recognized primarily as a complication of diabetes
- Better understanding of the role of the vagus nerve
- Focus on glucose control as primary treatment
- Limited recognition of non-diabetic forms
1990s-2000s: Neuromuscular Paradigm
- Recognition of the interstitial cells of Cajal as gastric pacemakers
- Better understanding of the enteric nervous system
- Development of more accurate diagnostic tools
- Introduction of electrical stimulation therapies
2010s-Present: Multifactorial Understanding
- Recognition of immune/inflammatory contributions
- Appreciation of the brain-gut axis in symptom generation
- Understanding of the role of gut microbiome
- Shift toward more personalized treatment approaches
- Recognition of gastroparesis as a complex syndrome rather than a single disease
- Emerging understanding of overlap with other functional gastrointestinal disorders
This evolution reflects a shift from viewing gastroparesis as simply a motility disorder to understanding it as a complex, multifactorial condition involving neuromuscular dysfunction, immune dysregulation, and altered sensation, with significant heterogeneity between patients.
3. Symptoms
Early Symptoms
In the initial stages, gastroparesis may present with subtle and intermittent symptoms that are often mistaken for other digestive conditions:
- Early satiety: Feeling full after eating only small amounts of food
- Postprandial fullness: Persistent fullness after meals that lasts longer than expected
- Upper abdominal discomfort: Typically non-painful fullness or pressure
- Mild nausea: Particularly after eating
- Occasional heartburn or regurgitation: Due to delayed gastric emptying
- Bloating: Sense of abdominal distension
- Fluctuating blood sugar levels: In diabetic patients, unexplained glucose variability
- Subtle changes in appetite: Gradually decreasing interest in food
- Mild food intolerances: Especially with fatty or fibrous foods
These early symptoms may come and go, often triggered by specific foods or stress, leading to delayed diagnosis as patients and clinicians may attribute them to dietary indiscretions or common conditions like dyspepsia.
Advanced-Stage Symptoms
As gastroparesis progresses, symptoms typically become more severe and persistent:
- Chronic, severe nausea: Often daily and debilitating
- Frequent vomiting: Sometimes of undigested food consumed many hours earlier
- Significant weight loss: Due to reduced intake and malabsorption
- Malnutrition: Results in fatigue, weakness, and vitamin deficiencies
- Severe abdominal pain: Becomes more common in advanced cases
- Erratic blood glucose control: In diabetic patients, severe and unpredictable fluctuations
- Bezoar formation: Accumulation of undigested food that forms a solid mass in the stomach
- Visible abdominal distension: Due to retained gastric contents
- Severe GERD symptoms: As gastric contents are more likely to reflux
- Small intestinal bacterial overgrowth (SIBO): Due to altered gut motility
- Significant reduction in quality of life: Inability to work or participate in social activities
Advanced gastroparesis can significantly impair quality of life and may lead to frequent hospitalizations for dehydration, malnutrition, and pain management.
Common vs. Rare Symptoms
Common Symptoms (affecting >50% of patients):
- Nausea (reported by up to 90% of patients)
- Early satiety (80%)
- Postprandial fullness (75%)
- Bloating (75%)
- Upper abdominal discomfort (70%)
- Vomiting (65%)
- Weight loss (50%)
Less Common Symptoms (affecting 20-50% of patients):
- Lower abdominal pain
- Constipation
- Heartburn
- Acid regurgitation
- Altered taste sensation
- Loss of appetite
Rare Symptoms (affecting <20% of patients):
- Cyclic pattern of symptoms with asymptomatic periods
- Dysgeusia (distorted taste)
- Globus sensation (feeling of a lump in the throat)
- Hypersalivation
- Severe, intractable pain as the primary symptom
- Nocturnal vomiting as the predominant symptom
- Rumination (regurgitation of undigested food without nausea or retching)
- Hiccups
- Non-gastrointestinal symptoms like orthostatic hypotension (in cases with broader autonomic dysfunction)
Symptom Progression Over Time
Gastroparesis typically follows one of several progression patterns:
1. Gradual Progressive Pattern:
- Begins with mild postprandial fullness and occasional nausea
- Gradually evolves to include more frequent nausea and episodes of vomiting
- Eventually leads to weight loss, nutritional deficiencies, and potential complications
- This pattern is common in diabetic gastroparesis
2. Post-Event Acute Onset Pattern:
- Sudden onset following a viral illness, surgery, or medication
- May begin with severe symptoms that gradually improve
- Some patients (30-50%) experience complete resolution within 12 months
- Others develop chronic symptoms despite resolution of the initial trigger
- Common in post-viral and post-surgical gastroparesis
3. Fluctuating Pattern:
- Alternating periods of significant symptoms and relative remission
- Flares may be triggered by stress, dietary indiscretions, or glycemic fluctuations
- Overall severity may remain stable or gradually worsen over time
- Common in idiopathic gastroparesis
4. Refractory Progressive Pattern:
- Begins with typical symptoms that respond poorly to standard interventions
- Steadily worsens despite treatment attempts
- May lead to severe malnutrition requiring enteral or parenteral nutrition
- Often associated with smooth muscle or ICC degeneration
Factors Influencing Progression:
- Diabetes control (in diabetic gastroparesis)
- Coexisting conditions (especially anxiety and depression)
- Medication effects
- Dietary compliance
- Access to specialized care
- Development of complications such as bezoars
- Response to initial treatments
Understanding the typical progression patterns helps clinicians anticipate potential complications and adjust management strategies accordingly. However, there remains significant variability between individuals, highlighting the importance of personalized monitoring and treatment approaches.
4. Causes
Biological Causes
The pathophysiology of gastroparesis involves complex interactions between neural, muscular, and hormonal elements of gastric function:
Neurological Factors:
- Vagal Nerve Dysfunction: The vagus nerve supplies parasympathetic innervation to the stomach and regulates motility; damage impairs coordinated contractions
- Enteric Nervous System Abnormalities: Alterations in the intrinsic nervous system of the digestive tract
- Autonomic Neuropathy: Broader dysfunction of the autonomic nervous system affecting gastric motility
- Central Nervous System Input: Altered central regulation of digestive function, particularly through the dorsal vagal complex
Cellular and Muscular Factors:
- Interstitial Cells of Cajal (ICC) Depletion: Loss of these gastric pacemaker cells is a hallmark finding
- Smooth Muscle Degeneration: Fibrosis or atrophy of gastric smooth muscle
- Immune Cell Infiltration: Particularly macrophages with abnormal secretory profiles
- Extrinsic Compression: Physical pressure on the stomach from surrounding structures
- Pyloric Dysfunction: Abnormal closure or spasm of the pyloric sphincter
- Impaired Fundic Accommodation: Inability of the stomach to relax appropriately to receive food
Biochemical and Molecular Mechanisms:
- Oxidative Stress: Molecular damage to gastric tissues through reactive oxygen species
- Advanced Glycation End Products: In diabetic patients, protein modifications that impair function
- Mitochondrial Dysfunction: Impaired cellular energy production
- Inflammatory Cytokines: Abnormal levels of TNF-α, IL-1β, IL-6 affecting gastric function
- Hormonal Imbalances: Alterations in motilin, ghrelin, GLP-1, and other gut peptides
- Insulin and Glucose Effects: Direct effects of glucose and insulin on gastric motility
Environmental Causes
External factors can trigger or exacerbate gastroparesis:
Medications:
- Opioid Analgesics: Directly inhibit gastric motility through multiple mechanisms
- Anticholinergics: Reduce acetylcholine-mediated gastric contractions
- GLP-1 Agonists: Used for diabetes but slow gastric emptying
- Tricyclic Antidepressants: Anticholinergic effects can impair motility
- Calcium Channel Blockers: Reduce smooth muscle contractility
- Dopamine Agonists: Can inhibit gastric motility
- Progesterone: May contribute to pregnancy-related gastroparesis
Infections:
- Viral Gastroenteritis: Particularly norovirus, rotavirus, and certain enteroviruses
- Post-infectious Immune Activation: Continued inflammation after infection resolution
- Bacterial Overgrowth: Secondary bacterial dysbiosis that further impairs motility
- Epstein-Barr Virus: Associated with some cases of post-viral gastroparesis
- COVID-19: Emerging evidence of post-COVID gastroparesis
Surgical and Procedural Factors:
- Vagal Nerve Damage: During esophageal, gastric, or gallbladder surgery
- Fundoplication: Can create mechanical and functional alterations
- Bariatric Surgery: Particularly restrictive procedures
- Radiation Therapy: To the abdomen or chest can damage neural and muscular components
- Traumatic Injury: Abdominal trauma affecting gastric innervation
Dietary and Lifestyle Factors:
- Severe Caloric Restriction: Can slow gastric emptying
- Chronic Alcohol Consumption: Direct toxic effects on vagal function and gastric muscle
- Tobacco Use: Impairs gastric blood flow and motility
- Extreme Psychological Stress: Through brain-gut axis signaling
Genetic and Hereditary Factors
Emerging evidence suggests genetic contributions to gastroparesis risk:
Known Genetic Associations:
- Mitochondrial DNA Mutations: Associated with diabetic gastroparesis
- Sodium Channel Mutations: SCN5A variations linked to gastroparesis risk
- NOD2 Polymorphisms: Associated with inflammatory pathway alterations
- Familial Visceral Myopathies: Rare inherited disorders affecting GI motility
- KIT Gene Mutations: Affecting ICC development and function
- Connective Tissue Disorder Genes: Such as those in Ehlers-Danlos syndrome
Potential Genetic Mechanisms:
- Predisposition to autoimmune responses targeting gastric tissues
- Altered neuronal nitric oxide synthase (nNOS) expression
- Variation in inflammatory response to tissue injury
- Differences in anti-oxidant defense systems
- Susceptibility to neuropathic damage from hyperglycemia
While specific genetic tests for gastroparesis risk are not yet clinically available, family history of unexplained digestive symptoms or confirmed gastroparesis may indicate increased risk.
Known Triggers and Exposure Risks
Certain factors can trigger onset or exacerbation of gastroparesis:
Acute Triggers:
- Hyperglycemia: Even transient blood sugar elevations can acutely slow gastric emptying
- Viral Illnesses: Particularly those affecting the gastrointestinal tract
- Major Surgery: Especially procedures involving the upper abdomen
- Psychological Stress: Acute severe stress can significantly slow gastric emptying
- Certain Foods: High-fat meals, carbonated beverages, and very fibrous foods
- New Medications: Particularly those listed in environmental causes
Chronic/Repetitive Exposures:
- Poorly Controlled Diabetes: Long-term hyperglycemia causes progressive damage
- Chronic Opioid Use: Progressive worsening with continued exposure
- Repetitive Vomiting: Can lead to gastric dysrhythmias that become self-perpetuating
- Cyclic Pattern of Starvation and Overeating: Disrupts normal gastric function
- Chronic Psychological Stress: Alters brain-gut signaling pathways
- Exposure to Environmental Toxins: Some pesticides and industrial chemicals
Understanding the complex interplay of these factors is essential for developing comprehensive prevention and treatment strategies. Many cases of gastroparesis involve multiple contributing factors rather than a single clear cause, highlighting the need for individualized assessment and management approaches.
5. Risk Factors
Demographic Risk Factors
Certain population groups have higher susceptibility to gastroparesis:
Age:
- Most commonly diagnosed between ages 20-50
- Peak incidence in the third and fourth decades of life
- Less commonly diagnosed in children and the elderly, though may be underreported in these groups
- Idiopathic gastroparesis particularly common in young to middle-aged adults
Gender:
- Strong female predominance with a female-to-male ratio of approximately 4:1
- Women account for approximately 80% of idiopathic gastroparesis cases
- The gender discrepancy is less pronounced in diabetic gastroparesis (approximately 60% female)
- Hormonal factors may contribute, as symptoms often worsen during the luteal phase of the menstrual cycle
Race/Ethnicity:
- Limited comprehensive epidemiological data exists on racial/ethnic variations
- Some studies suggest higher prevalence in Caucasian populations
- Potentially underdiagnosed in minority populations due to healthcare access disparities
- Genetic risk factors may vary between ethnic groups
Socioeconomic Factors:
- Higher diagnosis rates in areas with greater access to specialized healthcare
- Potential underdiagnosis in economically disadvantaged populations
- Challenges in management among those with limited healthcare resources
- Higher disease burden in those unable to afford specialized diets or medications
Lifestyle and Occupational Risk Factors
Certain behaviors and occupations may increase gastroparesis risk:
Dietary Patterns:
- Extremely low-calorie diets can slow gastric emptying
- Disordered eating patterns with cycles of restriction and bingeing
- Very high-fat diet consumption
- Excessive carbonated beverage intake
Substance Use:
- Chronic alcohol consumption
- Regular marijuana use (can cause cannabinoid hyperemesis syndrome, which shares features with gastroparesis)
- Tobacco use
- Illicit drug use, particularly opioids
Occupational Exposures:
- Healthcare workers with access to opioid medications
- Shift workers with disrupted circadian rhythms
- Occupations with extremely high stress levels
- Jobs with limited meal breaks, leading to irregular eating patterns
- Occupations with exposure to certain neurotoxic chemicals
- Factory workers exposed to heavy metals or industrial solvents
Other Lifestyle Factors:
- Sedentary lifestyle
- Poor sleep habits
- Chronic psychological stress
- Extreme athletic training (rare cases reported in endurance athletes)
Environmental and Genetic Factors
External and hereditary influences on gastroparesis risk:
Environmental Factors:
- Geographic regions with higher viral gastroenteritis rates
- Areas with limited access to specialized gastroenterology care
- Environmental toxin exposure in certain industrial regions
- Climate considerations (some patients report seasonal symptom variation)
Genetic Factors:
- Family history of gastroparesis or unexplained digestive symptoms
- Certain inherited connective tissue disorders (Ehlers-Danlos syndrome)
- Familial autonomic neuropathies
- Genetic predisposition to autoimmune conditions
- Hereditary mitochondrial disorders
- Congenital smooth muscle myopathies
- Family history of diabetes with neuropathic complications
Impact of Pre-existing Conditions
Several medical conditions significantly increase gastroparesis risk:
Major Risk-Associated Conditions:
Diabetes Mellitus: The leading identified cause of gastroparesis
- Type 1 diabetes carries higher risk than type 2
- Risk increases with duration of diabetes and poor glycemic control
- Approximately 40% of long-standing type 1 diabetics develop some degree of gastroparesis
Autoimmune Disorders:
- Scleroderma (systemic sclerosis)
- Sjögren’s syndrome
- Systemic lupus erythematosus
- Thyroiditis
- Multiple sclerosis
Neurological Conditions:
- Parkinson’s disease
- Multiple system atrophy
- Dysautonomias
- Spinal cord injuries
Metabolic/Endocrine Disorders:
- Hypothyroidism
- Chronic kidney disease
- Adrenal insufficiency
Gastrointestinal Disorders:
- Previous gastric surgery
- Inflammatory bowel disease
- Chronic intestinal pseudo-obstruction
- Chronic pancreatitis
Medication Use Associated with Increased Risk:
- Chronic opioid use
- Anticholinergic medications
- Some antidepressants
- Anti-Parkinson’s medications
- Calcium channel blockers
- GLP-1 receptor agonists for diabetes
- Some chemotherapy agents
Psychological Conditions:
- While psychological factors alone don’t cause gastroparesis, conditions like anxiety and depression are associated with:
- Increased symptom severity
- Delayed diagnosis
- Poorer treatment response
- Higher healthcare utilization
Understanding these risk factors helps identify individuals who may benefit from early screening, especially when presenting with suggestive symptoms. Additionally, modifiable risk factors offer opportunities for preventive interventions, particularly among high-risk populations such as those with long-standing diabetes.
6. Complications
Direct Complications of Gastroparesis
The impaired gastric emptying in gastroparesis can lead to several immediate complications:
Nutritional Complications:
- Malnutrition: Inadequate intake and malabsorption of essential nutrients
- Weight Loss: Often significant and difficult to reverse
- Vitamin Deficiencies: Particularly fat-soluble vitamins (A, D, E, K) and B vitamins
- Protein-Energy Malnutrition: In severe cases
- Sarcopenia: Loss of muscle mass and strength
- Micronutrient Deficiencies: Iron, zinc, calcium, magnesium, and others
Mechanical Complications:
- Bezoar Formation: Hardened masses of undigested food that can cause obstruction
- Phytobezoars (plant material)
- Trichobezoars (hair, more common in psychiatric patients)
- Mixed composition bezoars
- Gastric Dilatation: Abnormal enlargement of the stomach
- Gastric Perforation: Rare but life-threatening complication of severe distension
- Gastric Volvulus: Twisting of the stomach (rare)
- Small Bowel Bacterial Overgrowth (SIBO): Due to stasis and altered gut motility
Metabolic Complications:
- Glycemic Control Difficulties: In diabetic patients
- Unpredictable blood glucose fluctuations
- Increased hypoglycemic events
- Difficulty managing insulin dosing
- Electrolyte Imbalances: Due to vomiting and poor intake
- Hypokalemia
- Hyponatremia
- Hypochloremic metabolic alkalosis
- Dehydration: Often requiring intravenous rehydration
- Acid-Base Disturbances: From chronic vomiting
Long-term Impact on Overall Health
Gastroparesis can affect multiple body systems over time:
Gastrointestinal System:
- Chronic GERD: With risk of Barrett’s esophagus
- Esophagitis: From repeated vomiting
- Altered Gut Microbiome: With wide-ranging health implications
- Gastric Atrophy: From chronic inflammation and disuse
- Dental Erosion: From acid exposure during vomiting
- Progression of Motility Disorders: To involve other GI organs
Cardiovascular System:
- Orthostatic Hypotension: Due to volume depletion and autonomic dysfunction
- Cardiac Arrhythmias: From electrolyte disturbances
- Increased Risk of QT Prolongation: Both from the condition and medications used to treat it
Endocrine System:
- Worsening Diabetes Control: Creating a vicious cycle
- Impaired Medication Absorption: Affecting treatment of comorbid conditions
- Disrupted Hunger/Satiety Signaling: With metabolic consequences
Renal System:
- Chronic Kidney Disease Risk: From recurrent dehydration
- Nephrolithiasis (Kidney Stones): Associated with chronic dehydration and metabolic changes
- Medication Clearance Issues: Affecting dosing of renally excreted drugs
Immune System:
- Increased Infection Susceptibility: Due to malnutrition
- Delayed Wound Healing: From nutritional deficiencies
- Potential Autoimmune Interactions: Particularly in those with underlying autoimmune disorders
Central Nervous System:
- Cognitive Effects: From malnutrition and electrolyte disturbances
- Mood Disorders: Both as a result and exacerbating factor of gastroparesis
- Sleep Disturbances: Due to symptoms and medications
- Chronic Pain Syndromes: Development of central sensitization
Psychological and Quality of Life Impact
The chronic nature of gastroparesis creates significant psychological burden:
- Depression: Affects up to 50% of gastroparesis patients
- Anxiety: Particularly related to eating and social situations
- Social Isolation: Due to unpredictable symptoms and dietary restrictions
- Reduced Work Productivity: 30-40% report significant work impairment
- Financial Burden: From healthcare costs and decreased earning capacity
- Eating-Related Distress: Development of food fears and aversions
- Body Image Issues: Related to weight loss, feeding tubes, or devices
- Relationship Difficulties: Impact on family dynamics and intimate relationships
- “Invisible Illness” Burden: Lack of understanding from others
Potential Disability or Fatality
Severe gastroparesis can lead to significant disability and life-threatening complications:
Disability Aspects:
- Approximately 18-25% of patients with gastroparesis become disabled
- Up to 40% of patients with severe gastroparesis cannot maintain regular employment
- Many require accommodations under disability legislation
- Activities of daily living often significantly compromised
- Home-bound status in severe cases
Hospitalization and Healthcare Utilization:
- Average gastroparesis patient has 2-3 times higher healthcare utilization than age-matched controls
- 30-40% of patients require hospitalization annually for symptom management
- Average hospital stay for gastroparesis complications: 4-6 days
- Frequent emergency department visits for acute symptom flares
- Some patients require repeated hospitalizations for nutrition and hydration
Severe Outcomes and Mortality:
- Mortality directly attributable to gastroparesis is rare but does occur
- Most common causes of death related to gastroparesis:
- Complications of malnutrition
- Aspiration pneumonia
- Complications from total parenteral nutrition
- Electrolyte disturbances leading to cardiac events
- Severe bezoar-related complications
- Standardized mortality ratio approximately 1.5 times higher than age-matched controls
- Life expectancy may be reduced by 5-10 years in severe, refractory cases
Factors Associated with Poorer Outcomes:
- Diabetic etiology with poor glycemic control
- Delayed diagnosis (>2 years from symptom onset)
- Male gender (though less common, often more severe)
- Presence of autonomic neuropathy affecting multiple systems
- Inadequate access to specialized care
- Requirement for jejunal feeding or parenteral nutrition
- Development of narcotic dependence for pain management
- Failure of multiple treatment modalities
While most patients with gastroparesis can achieve reasonable symptom control with appropriate management, a subset with severe, refractory disease faces significant disability and potentially life-threatening complications. This underscores the importance of early diagnosis, multidisciplinary management, and continued research into more effective treatments.
7. Diagnosis & Testing
Initial Evaluation and Assessment
The diagnostic journey for gastroparesis typically begins with a comprehensive clinical evaluation:
Medical History:
- Detailed symptom assessment using validated tools such as the Gastroparesis Cardinal Symptom Index (GCSI)
- Temporal relationship of symptoms to meals
- Dietary habits and food triggers
- Medication review
- History of diabetes, surgeries, or viral illnesses
- Family history of gastrointestinal disorders
- Previous treatments and their effectiveness
Physical Examination:
- Assessment of nutritional status and hydration
- Abdominal examination for distension, tenderness, or succussion splash
- Signs of autonomic neuropathy
- Fundoscopic examination in diabetic patients
- Dental evaluation for signs of chronic vomiting
- Neurological assessment if neuropathy is suspected
Initial Laboratory Testing:
- Complete blood count to assess for anemia
- Comprehensive metabolic panel (including electrolytes, renal and hepatic function)
- Thyroid function tests
- HbA1c and fasting glucose in non-diabetic patients
- Amylase and lipase to rule out pancreatitis
- Pregnancy test in women of childbearing age
- Celiac disease screening
Diagnostic Tests for Gastroparesis
Several specialized tests are used to confirm the diagnosis and assess severity:
Gold Standard: Gastric Emptying Studies
Gastric Emptying Scintigraphy (GES):
- Standard 4-hour protocol using a radiolabeled solid meal
- Measures percentage of meal retained at 1, 2, and 4 hours
- Diagnosis established when >60% retention at 2 hours or >10% at 4 hours
- Advantages: Standardized, quantitative, physiological
- Limitations: Radiation exposure, availability, standardization between centers
13C-Breath Testing:
- Non-radioactive alternative using carbon-13 labeled substrates
- Measures CO2 exhalation rate as an indirect measure of gastric emptying
- Advantages: No radiation, can be performed in office setting
- Limitations: Less well-standardized, indirect measurement
Wireless Motility Capsule (SmartPill):
- Ingestible capsule that records pH, temperature, and pressure
- Provides gastric emptying time and whole gut transit information
- Advantages: Comprehensive assessment, ambulatory
- Limitations: Cost, availability, contraindicated with strictures
Adjunctive Diagnostic Studies:
Upper Endoscopy (EGD):
- Essential to rule out mechanical obstruction and other conditions
- May reveal retained food despite fasting
- Allows for tissue sampling to exclude other conditions
- Not diagnostic for gastroparesis but necessary in the workup
Antroduodenal Manometry:
- Measures pressure patterns of stomach and small intestine contractions
- Helps differentiate neuropathic from myopathic motility disorders
- Useful in treatment-refractory cases
- Limited availability to specialized centers
Electrogastrography (EGG):
- Non-invasive recording of gastric electrical activity using cutaneous electrodes
- Can identify gastric dysrhythmias associated with gastroparesis
- Primarily used in research settings with limited clinical application
Gastric Barostat Study:
- Measures gastric accommodation and sensitivity
- Helpful in distinguishing gastroparesis from functional dyspepsia
- Limited availability to research centers
Advanced Imaging:
- MRI for gastric volume and emptying assessment (emerging technique)
- Ultrasound for gastric emptying (limited standardization)
- CT scan to rule out complications or other conditions
Specialized Testing
For complex or unclear cases, additional testing may be warranted:
Autonomic Function Testing:
- Used when autonomic neuropathy is suspected
- Includes tilt-table testing, sudomotor assessment, and cardiovagal testing
- Helps establish the extent of autonomic involvement
Gastric Full-Thickness Biopsy:
- Obtained surgically or endoscopically
- Examines ICC networks, neural plexuses, and smooth muscle
- Reserved for refractory cases where etiology remains unclear
- Can guide treatment decisions, particularly regarding surgical options
Genetic Testing:
- Emerging field with limited clinical application currently
- May be considered in familial cases or those with suspected syndromic disorders
- Not routinely recommended outside research protocols
Screening for Associated Conditions:
- Small bowel bacterial overgrowth testing
- Pancreatic exocrine function testing
- Advanced glycation end-product assessment in diabetic patients
- Autoimmune panels in suspected immune-mediated cases
Differential Diagnosis
Several conditions mimic gastroparesis and must be excluded:
Mechanical Obstructions:
- Pyloric stenosis
- Malignancy
- Peptic ulcer disease with outlet obstruction
- External compression from adjacent organs
- Annular pancreas
Functional Disorders:
- Functional dyspepsia
- Rumination syndrome
- Cyclic vomiting syndrome
- Chronic nausea and vomiting syndrome
Metabolic/Systemic Conditions:
- Chronic pancreatitis
- Celiac disease
- Hypothyroidism
- Adrenal insufficiency
- Electrolyte disorders
Neurological Disorders:
- Central nervous system lesions affecting vagal nuclei
- Intracranial hypertension
- Vestibular disorders
- Migraine variants
Other Considerations:
- Medication effects
- Eating disorders
- Psychogenic vomiting
- Cannabis hyperemesis syndrome
- Superior mesenteric artery syndrome
Early Detection Methods and Effectiveness
Identifying gastroparesis in its early stages can significantly improve outcomes:
Screening High-Risk Populations:
- Diabetes patients with unexplained glucose variability
- Post-surgical patients with persistent digestive symptoms
- Patients after viral gastroenteritis who have lingering symptoms
- Individuals with unexplained weight loss and early satiety
Early Detection Approaches:
- Standardized symptom questionnaires (GCSI-Daily Diary)
- Point-of-care 13C-breath testing in primary care settings
- Incorporation of gastroparesis screening into routine diabetes care
- Gastric emptying scintigraphy with nutrient challenge in borderline cases
Effectiveness of Early Detection:
- Earlier diagnosis associated with:
- Better response to dietary modifications
- More effective symptom management
- Reduced risk of nutritional deficiencies
- Lower rate of hospitalization
- Better preservation of quality of life
- Challenges in implementation:
- Symptom overlap with common conditions
- Limited awareness among primary care providers
- Resource constraints for specialized testing
- Patient delay in seeking care for “vague” symptoms
The diagnostic approach to gastroparesis requires systematic exclusion of other conditions while establishing objective evidence of delayed gastric emptying. A combination of standardized gastric emptying studies, endoscopy, and targeted additional testing based on clinical presentation typically yields the most accurate diagnosis. Early detection in high-risk populations remains an important but challenging goal in improving overall outcomes.
8. Treatment Options
Dietary and Nutritional Management
Dietary modifications form the first-line approach for most gastroparesis patients:
Dietary Principles:
- Small, Frequent Meals: 6-8 small meals instead of 3 large ones
- Low-Fat Content: Fat delays gastric emptying
- Low-Fiber Approach: Fiber can be difficult to empty from the stomach
- Adequate Hydration: Emphasis on non-carbonated, non-caffeinated fluids
- Semi-Solid or Liquid Nutrition: Easier to empty than solids
- Thorough Food Mastication: Chewing food extensively before swallowing
- Limiting Raw Fruits and Vegetables: Often poorly tolerated
- Post-Meal Posture: Sitting or walking after meals to utilize gravity
Specific Dietary Recommendations:
- Well-Tolerated Foods:
- White bread, crackers, and plain pasta
- Lean protein sources (baked or grilled)
- Cooked, low-fiber vegetables
- Fruit and vegetable juices without pulp
- Low-fat dairy or alternatives
- Nutritional supplement drinks
- Foods Typically Poorly Tolerated:
- High-fat meats and dairy
- Fried foods
- Raw vegetables and fruits with skins
- Nuts and seeds
- Carbonated beverages
- Alcohol
- High-fiber whole grains
Nutritional Support Strategies:
- Oral Nutritional Supplements: Liquid meal replacements
- Vitamin and Mineral Supplementation: Based on identified deficiencies
- Enteral Nutrition: For patients unable to maintain nutrition orally
- Nasojejunal tubes for short-term support
- Jejunostomy tubes for long-term nutrition
- Parenteral Nutrition: Reserved for cases where enteral nutrition is inadequate or not tolerated
Pharmacological Treatments
Several medication classes are used to manage gastroparesis symptoms:
Prokinetic Agents (enhance gastric motility):
Metoclopramide:
- D2 receptor antagonist and 5-HT4 agonist
- FDA-approved for short-term use (up to 12 weeks)
- Efficacy: 30-40% symptom improvement
- Limitations: Neurological side effects, tardive dyskinesia risk with long-term use
Domperidone:
- D2 receptor antagonist
- Available in US only through FDA expanded access programs
- Efficacy: Similar to metoclopramide but fewer central nervous system effects
- Limitations: QT prolongation risk, limited availability
Erythromycin:
- Motilin receptor agonist
- Most effective for short-term use
- Efficacy: 30-60% acute symptom improvement
- Limitations: Tachyphylaxis, antibiotic resistance concerns, QT prolongation
Prucalopride:
- 5-HT4 receptor agonist
- FDA approved for chronic constipation, used off-label for gastroparesis
- Efficacy: 30-45% improvement in early studies
- Limitations: Cost, limited long-term data
Antiemetic Medications:
- Phenothiazines (prochlorperazine, chlorpromazine)
- 5-HT3 Antagonists (ondansetron, granisetron)
- NK1 Receptor Antagonists (aprepitant)
- Antihistamines (diphenhydramine, meclizine)
- Cannabinoids (dronabinol, medical marijuana where legal)
- Benzodiazepines: For anxiety-associated nausea
Pain Management:
- Tricyclic Antidepressants: Low-dose amitriptyline, nortriptyline
- Gabapentinoids: Gabapentin, pregabalin
- Selective Serotonin Reuptake Inhibitors (SSRIs)
- Transdermal Lidocaine
- Opioid Management: Complicated by potential to worsen gastroparesis
- Alpha-lipoic Acid: For neuropathic pain components
Other Medication Approaches:
- Pyloric Botulinum Toxin Injection: Transient benefit in some patients
- Ghrelin Agonists: Under investigation (relamorelin, ulimorelin)
- Anti-inflammatory Medications: For suspected immune-mediated cases
- Mirtazapine: Antiemetic, appetite stimulant, and mood stabilizer
Endoscopic and Surgical Interventions
When conservative approaches fail, several interventional options exist:
Endoscopic Approaches:
- Pyloric Botulinum Toxin Injection:
- Injection into the pyloric sphincter
- Temporary effect lasting 3-6 months
- Controversial efficacy in controlled trials
- May help identify candidates for more permanent interventions
- Gastric Per-Oral Endoscopic Myotomy (G-POEM):
- Endoscopic technique to divide the pyloric muscle
- Success rates of 60-80% in carefully selected patients
- Emerging technique with growing evidence base
- Less invasive than surgical pyloroplasty
- Endoscopic Pyloromyotomy:
- Various techniques including balloon dilation
- Variable success rates (40-70%)
- Generally lower efficacy than surgical approaches
- Stent Placement:
- Transpyloric stenting to maintain pyloric opening
- Limited by migration and long-term complications
- Typically used as a bridge to more definitive therapy
Surgical Interventions:
Gastric Electrical Stimulation (GES):
- Implantable device delivering low-energy stimulation to the stomach
- FDA approved as a Humanitarian Use Device
- Efficacy: 50-60% of patients experience significant improvement
- Mechanism may involve afferent vagal effects rather than direct motility enhancement
Pyloroplasty:
- Surgical widening of the pyloric channel
- Often combined with gastric electrical stimulation
- Higher efficacy than endoscopic approaches but more invasive
Subtotal Gastrectomy:
- Removal of portion of the stomach with reconstruction
- Reserved for severe, refractory cases
- High efficacy but significant risk profile
Gastric Bypass:
- Particularly in diabetic gastroparesis with obesity
- Addresses both weight and gastric emptying
- Emerging data supports efficacy in selected patients
Total Gastrectomy:
- Complete stomach removal
- Last-resort option for intractable symptoms
- Significant long-term nutritional implications
Feeding Tube Placement:
- Jejunostomy tubes bypass the dysfunctional stomach
- Enable enteral nutrition despite gastroparesis
- Can be temporary or permanent depending on clinical course
Complementary and Alternative Approaches
Several non-conventional approaches show promise for symptom management:
Mind-Body Approaches:
- Cognitive Behavioral Therapy: Addresses anxiety, pain catastrophizing, and coping
- Hypnotherapy: Demonstrated efficacy for functional GI disorders
- Biofeedback: Particularly for associated pelvic floor dysfunction
- Mindfulness-Based Stress Reduction: Improves coping and may reduce symptom perception
- Relaxation Techniques: Progressive muscle relaxation, guided imagery
Manual and Physical Therapies:
- Acupuncture/Acupressure: Some evidence for nausea reduction
- Massage Therapy: May help with associated abdominal pain
- Physical Therapy: For associated musculoskeletal issues
- Osteopathic Manipulative Treatment: Limited evidence for visceral techniques
Supplementary Approaches:
- Ginger: Natural prokinetic and antiemetic properties
- Peppermint: May reduce nausea and pain
- Probiotics: Evidence emerging for specific strains
- Traditional Chinese Medicine: Herbal formulations with limited evidence
- Iberogast: Herbal preparation with prokinetic properties
Emerging Treatments and Clinical Trials
The therapeutic landscape for gastroparesis continues to evolve:
Pharmacological Innovations:
- Relamorelin: Ghrelin receptor agonist in late-stage trials
- Tradipitant: NK1 receptor antagonist showing promise in clinical trials
- Velusetrag: 5-HT4 receptor agonist under investigation
- TAK-906: D2/D3 receptor antagonist with limited CNS penetration
- Selective Muscarinic Agonists: M1 and M3 receptor targets
- Tegaserod: Recently reintroduced with potential gastroparesis applications
Device and Procedural Innovations:
- Minimally Invasive Vagal Nerve Modulation:
- Transcutaneous auricular vagal stimulation
- Minimally invasive vagal nerve stimulators
- Next-Generation Gastric Electrical Stimulation:
- Adaptive stimulation parameters
- Meal-triggered stimulation patterns
- Wireless power systems
- Novel Endoscopic Techniques:
- Submucosal ablation of the pylorus
- POEM variations with enhanced safety profiles
- Endoscopic gastric plication
Regenerative Medicine Approaches:
- Stem Cell Therapy: ICC restoration through various approaches
- Enteric Nervous System Regeneration: Neuronal growth factors
- Bioelectronic Medicine: Targeted organ-specific neuromodulation
- 3D Bioprinted Gastric Tissues: Early experimental phase
Multidisciplinary Treatment Programs:
- Comprehensive centers integrating nutritional, psychological, and medical approaches
- Telehealth models for extending specialized care to underserved regions
- Patient-led peer support integrated with medical management
A comprehensive management approach typically involves combining dietary modifications, appropriate medications, psychological support, and consideration of more invasive interventions in refractory cases. The treatment plan must be individualized based on gastroparesis etiology, symptom profile, nutritional status, and patient preferences. Emerging therapies continue to offer hope for improved outcomes in this challenging condition.
9. Prevention & Precautionary Measures
Primary Prevention Strategies
While not all cases of gastroparesis are preventable, several strategies may reduce risk:
Diabetes Management:
- Tight Glycemic Control: Maintaining blood glucose within target ranges
- Early and Aggressive Diabetes Treatment: Preventing cumulative nerve damage
- Regular Screening: Early detection of autonomic neuropathy
- Medication Management: Avoiding agents that slow gastric emptying when possible
- Comprehensive Diabetes Education: Understanding the GI complications of diabetes
Post-Surgical Considerations:
- Nerve-Sparing Surgical Techniques: Particularly for upper abdominal procedures
- Vagus Nerve Identification and Preservation: During fundoplication, bariatric surgery
- Minimally Invasive Approaches: When appropriate and technically feasible
- Post-Operative Prokinetics: Prophylactic use in high-risk cases
- Early Mobilization After Surgery: Promotes return of GI function
Medication Awareness:
- Opioid Minimization: Using alternative pain management strategies when possible
- Medication Review: Regular assessment of medications that may affect gastric emptying
- Proactive Management: When high-risk medications cannot be avoided
- Drug Timing Optimization: Administering medications to minimize impact on digestion
- Pharmacist Involvement: In medication regimen assessment
Viral Illness Management:
- Prompt Treatment of Gastroenteritis: Early supportive care
- Hydration Maintenance: During acute gastrointestinal illnesses
- Graduated Return to Normal Diet: After acute GI infections
- Temporary Prokinetic Support: During recovery phase when appropriate
Secondary Prevention (Management of Early Disease)
Early intervention may prevent progression to severe gastroparesis:
Early Symptom Recognition and Assessment:
- Public and provider education about early warning signs
- Validation of brief screening tools for primary care settings
- Lower threshold for testing in high-risk populations
- Recognition of “silent” gastroparesis (asymptomatic delays in gastric emptying)
Prompt Intervention for Mild Symptoms:
- Dietary modifications before significant nutritional compromise occurs
- Early prokinetic therapy for symptomatic delays
- Addressing psychological aspects from disease onset
- Glycemic optimization at first signs of gastric function alterations
Multidisciplinary Approach from Disease Onset:
- Nutritionist involvement at diagnosis
- Psychological support integration into initial management plan
- Physical therapy assessment for associated musculoskeletal issues
- Appropriate specialist referrals based on etiology
Lifestyle Modifications for Early Disease:
- Meal spacing and composition optimization
- Activity adjustments to facilitate gastric emptying
- Stress management techniques
- Sleep hygiene improvement
Lifestyle and Environmental Considerations
Several lifestyle factors can help manage risk and symptoms:
Dietary Approaches:
- Meal Pattern Optimization:
- Small, frequent meals
- Consistent eating schedule
- Adequate but not excessive fluid intake
- Limiting food consumption before bedtime
- Food Preparation Methods:
- Mechanical soft diet when needed
- Pureeing or blending difficult foods
- Cooking vegetables thoroughly
- Using low-fat cooking methods
- Strategic Nutrient Timing:
- Liquid nutrition when symptoms are severe
- More substantial meals during symptom-free periods
- Distributing protein intake throughout the day
- Timing carbohydrates for optimal glucose control in diabetics
Physical Activity Considerations:
- Post-Meal Activity:
- Gentle walking after eating
- Avoiding immediate recumbent position
- Mild physical activity to promote gastric emptying
- Regular Exercise Regimen:
- Maintaining overall fitness within limitations
- Core strength development
- Avoiding exercises that increase intra-abdominal pressure
- Timing exercise relative to meals
Environmental Factors:
- Temperature Regulation: Avoiding extreme heat that may exacerbate dehydration
- Workplace Accommodations: Meal break flexibility, bathroom access
- Travel Planning: Medication and food availability during trips
- Social Environment: Education of family and friends about condition requirements
Special Population Considerations
Preventive approaches may differ for specific populations:
Children and Adolescents:
- Earlier intervention for diabetic youth
- Age-appropriate education about symptom recognition
- Working with schools on meal and bathroom accommodations
- Family-based approach to management
Elderly Patients:
- Medication review with particular attention to anticholinergic burden
- Assessment of swallowing function and aspiration risk
- Addressing comorbidities that may exacerbate symptoms
- Nutritional interventions suitable for age-related changes
Pregnant Women:
- Pre-conception counseling for women with known gastroparesis
- Proactive management of nausea and vomiting of pregnancy
- Nutritional support strategies safe during pregnancy
- Post-delivery monitoring for symptom resolution
Post-Surgical Patients:
- Targeted prevention for high-risk surgical procedures
- Prophylactic prokinetics in selected cases
- Early enteral nutrition when appropriate
- Graduated diet advancement plans
Patients with Autoimmune Conditions:
- Vigilance for gastroparesis symptoms
- Consideration of GI involvement in treatment plans
- Integration of gastroparesis prevention into overall disease management
- Early testing when suggestive symptoms emerge
Monitoring and Surveillance
Regular assessment can identify problems before they become severe:
For High-Risk Individuals:
- Diabetic Patients:
- Annual symptom questionnaire
- Gastric emptying testing when symptoms emerge
- Regular nutritional assessment
- Monitoring for medication effectiveness and side effects
- Post-Surgical Patients:
- Scheduled follow-up focused on GI function
- Early intervention for persistent symptoms
- Nutritional monitoring during recovery
- Patients on High-Risk Medications:
- Regular reassessment of medication necessity
- Symptom monitoring
- Consideration of drug holidays when possible
Monitoring Technology:
- Continuous glucose monitoring in diabetic gastroparesis
- Smartphone applications for symptom tracking
- Remote patient monitoring for high-risk individuals
- Wearable technology for hydration and nutritional monitoring
While complete prevention of gastroparesis is not always possible, these strategies can reduce risk, facilitate early detection, and prevent progression to more severe disease. The most effective approach combines general preventive measures with targeted strategies for specific high-risk populations. Additionally, early and appropriate intervention when symptoms first appear may prevent the development of complications and refractory disease.
10. Global & Regional Statistics
Global Prevalence and Incidence
Gastroparesis epidemiology varies significantly worldwide:
Overall Global Burden:
- Estimated Global Prevalence: 1-2% of the general population
- Annual Incidence: 6.3-15.8 new cases per 100,000 person-years
- Diagnosed Cases: Likely represent only 20-30% of actual cases due to underdiagnosis
- Growth Trend: Increasing recognition and diagnosis worldwide
Geographic Variations:
- North America: Highest reported prevalence and diagnosis rates
- United States: Approximately 10 cases per 100,000 person-years
- Canada: Similar to US rates
- Europe:
- Northern Europe: 4-6 cases per 100,000 person-years
- Southern Europe: Lower reported rates, likely due to underdiagnosis
- Asia:
- Japan: 2.4 cases per 100,000 person-years
- China: Limited data, estimated 1-3 cases per 100,000
- India: Higher rates in diabetic populations, limited general population data
- Australia/New Zealand: 7-9 cases per 100,000 person-years
- Africa and South America: Very limited epidemiological data available
Variations by Etiology:
- Diabetic Gastroparesis:
- Higher in regions with greater diabetes prevalence
- North America and Middle East show highest rates
- Post-Surgical Gastroparesis:
- More common in regions with higher rates of upper GI surgeries
- Japan reports higher proportions due to gastric cancer surgery rates
- Idiopathic Gastroparesis:
- Represents largest proportion in Western countries
- Less recognized in developing regions
Demographics and Distribution
Gastroparesis affects different populations at varying rates:
Age Distribution:
- Peak diagnosis age: 30-50 years
- Pediatric gastroparesis: Accounts for approximately 5% of cases
- Elderly (>65 years): Rising prevalence but often underdiagnosed
- Age-adjusted incidence increasing in all age groups
Gender Distribution:
- Female predominance: 70-80% of cases
- Female-to-male ratio approximately 4:1 for idiopathic gastroparesis
- More equal gender distribution in diabetic gastroparesis (60:40 F:M)
- Male patients often present with more severe disease despite lower prevalence
Socioeconomic Patterns:
- Higher diagnosis rates in countries with advanced healthcare systems
- Urban vs. rural: Higher diagnosed prevalence in urban areas with specialized care
- Socioeconomic disparities in access to specialized testing and care
- Higher hospitalization rates among lower-income populations
Racial/Ethnic Considerations:
- Limited comprehensive data on racial/ethnic variations
- Some studies suggest higher prevalence in Caucasian populations
- African Americans may have higher hospitalization rates when diagnosed
- Native American populations show higher rates of diabetic gastroparesis
Mortality and Morbidity Statistics
Impact on survival, hospitalization, and quality of life:
Mortality Data:
- Direct mortality: Rare, estimated at <1% annually
- Standardized mortality ratio: 1.5 times age-matched controls
- Causes of death: Typically complications of malnutrition or aspiration
- Life expectancy impact: 5-10 year reduction in severe, refractory cases
Hospitalization Burden:
- Annual hospitalizations:
- United States: Approximately 15,000 primary discharge diagnoses of gastroparesis
- Estimated 120,000 hospitalizations where gastroparesis is a contributing factor
- Readmission rates: 30-day readmission in 20-35% of cases
- Average length of stay: 4-6 days
- Annual cost of hospitalizations: $2.1-$3.8 billion in US alone
Emergency Department Utilization:
- 30-40% of diagnosed patients visit emergency departments annually
- Average of 2-3 ED visits per patient per year
- Higher among those with limited access to specialized outpatient care
Work and Disability Impact:
- 15-25% of patients report being unable to work due to symptoms
- Approximately 30% report reduced work hours or productivity
- Disability claims related to gastroparesis increasing at 2-3% annually
- Average work productivity loss estimated at 30-50%
Regional Trends and Patterns
Notable differences in presentation and management across regions:
North America:
- Highest healthcare utilization and cost
- Most established specialty centers and research programs
- Greater use of device-based therapies like gastric electrical stimulation
- Higher rates of opioid use for pain management
- Growing awareness and patient advocacy movements
Europe:
- More conservative approach to diagnosis and management
- Greater emphasis on dietary and lifestyle interventions
- More restricted use of medications (particularly in UK and Scandinavia)
- Regional variations in access to specialized care (North-South gradient)
- Different regulatory environment affecting available treatments
Asia:
- Different predominant etiologies (more post-surgical, less idiopathic)
- Alternative medicine more commonly integrated into treatment
- Different dietary approaches based on cultural food practices
- Less utilization of feeding tubes and parenteral nutrition
- Emerging recognition and specialized care development
Australia/New Zealand:
- Combined approaches drawing from both US and European models
- Strong emphasis on multidisciplinary management
- Geographic challenges in providing specialized care to remote areas
- Active research programs despite smaller population
Developing Regions:
- Limited access to standard diagnostic testing
- Focus on managing diabetic gastroparesis as diabetes rates rise
- Resource limitations affecting treatment options
- Reliance on dietary management and available medications
- Growing awareness among medical communities
Economic Impact and Healthcare Utilization
The financial burden of gastroparesis varies globally:
Direct Healthcare Costs:
- United States: Average annual cost per patient: $18,000-$27,000
- Europe: Varies by country, averaging €9,000-€15,000 annually
- Canada: Approximately CAD $12,000-$18,000 per patient annually
- Australia: AUD $10,000-$16,000 per patient annually
Indirect Costs:
- Lost productivity: Estimated $5,000-$15,000 per patient annually
- Caregiver burden: Often unquantified but substantial
- Disability payments: Varying by country’s social support system
- Transportation and accommodation for specialized care
Cost Distribution:
- Hospitalization: 40-60% of total costs
- Medications: 15-25% of costs
- Outpatient care: 10-20% of costs
- Diagnostic testing: 5-10% of costs
- Devices and surgical interventions: Highly variable
Healthcare Resource Utilization:
- Specialist visits: Average 4-8 gastroenterology visits annually
- Primary care visits: 6-12 annually
- Endoscopic procedures: 0.5-2 annually
- Emergency services: 2-3 visits annually in 30-40% of patients
The global and regional statistics highlight significant variations in gastroparesis epidemiology, recognition, and management approaches. The true global burden is likely underestimated due to limited data from many regions and widespread underdiagnosis. Economic impact is substantial, with both direct healthcare costs and indirect costs from lost productivity contributing to the overall burden.
11. Recent Research & Future Prospects
Latest Advancements in Understanding
Pathophysiological Insights:
Immune-Mediated Mechanisms:
- Recently identified “gastroparesis-specific macrophage phenotype”
- Discovery of M1/M2 macrophage imbalance in gastroparesis tissue
- Recognition of mast cell involvement in some patients
- Potential autoimmune etiology in a subset of cases
Microbiome Research:
- Altered gastric and small intestinal microbiome profiles in gastroparesis
- Bacterial overgrowth contributing to symptom generation
- Potential therapeutic implications of microbiome modulation
- Metabolomic changes associated with altered gut flora
Genetic and Molecular Discoveries:
- Identification of SCN5A sodium channel mutations associated with gastroparesis
- Mitochondrial DNA variations linked to diabetic gastroparesis susceptibility
- MicroRNA profiles differentiating gastroparesis subtypes
- Proteomic signatures of gastroparesis identified in blood and tissue
Advanced Imaging and Functional Studies:
- High-resolution 3D imaging of the enteric nervous system
- Molecular imaging of ICC networks
- Functional MRI studies of gastric motility
- Novel tracers for PET imaging of gastric innervation
Recent Treatment Innovations
Pharmacological Developments:
- Ghrelin Receptor Agonists:
- Relamorelin: Phase 3 trials showing promising results for diabetic gastroparesis
- Improved gastric emptying and symptom reduction without central effects
- Target dosing and administration protocols being refined
- Novel Prokinetics:
- Velusetrag: 5-HT4 agonist with improved safety profile
- TAK-906: D2/D3 antagonist with minimal central nervous system penetration
- Prucalopride: Repurposed from chronic constipation treatment
- Anti-Inflammatory Approaches:
- Targeted anti-cytokine therapies based on tissue analysis
- Low-dose naltrexone for immune modulation
- Mast cell stabilizers for selected patients
- Neuromodulation Medications:
- Tradipitant: NK1 receptor antagonist showing promise in clinical trials
- Novel GABA modulators for visceral hypersensitivity
- Vagal afferent targeting compounds
Interventional Advances:
- Endoscopic Innovations:
- Per-oral Pyloromyotomy (G-POEM): Refinement of technique and patient selection
- Endoscopic sleeve gastroplasty for gastroparesis with obesity
- Transpyloric stenting techniques with improved stent designs
- Submucosal endoscopy with tunneling for access to gastric muscular layers
- Device-Based Therapies:
- Next-generation gastric electrical stimulators with adaptive algorithms
- External non-invasive gastric stimulation techniques
- Implantable pressure sensors for real-time monitoring
- Vagal nerve stimulation with improved targeting
- Minimally Invasive Surgical Approaches:
- Laparoscopic pyloroplasty techniques with reduced morbidity
- Combined procedures addressing multiple pathophysiological components
- Robotic-assisted surgery with enhanced precision
- Adjustable gastric restriction devices for refractory symptoms
Complementary and Integrative Approaches:
- Targeted Nutritional Therapies:
- Specialized formulations designed for gastroparesis
- Medium-chain triglyceride supplementation
- Micronutrient optimization protocols
- Anti-inflammatory dietary protocols
- Mind-Body Medicine:
- Structured gut-directed hypnotherapy protocols
- Virtual reality-based therapy for pain management
- Specialized cognitive behavioral therapy programs
- Digital health applications for symptom management
Ongoing Clinical Trials and Research Initiatives
Major Ongoing Trials:
- GLOW Study: Multi-center evaluation of relamorelin for diabetic gastroparesis
- RETREAT Trial: Randomized evaluation of transcutaneous vagal stimulation
- DIGNITY: Diabetic gastroparesis intervention with targeted immune therapy
- PyloMyo: Comparative effectiveness of G-POEM versus surgical pyloromyotomy
- PROGRESS: Patient-reported outcomes and gastric emptying correlation study
- RESTORE-GP: Microbiome restoration therapy for gastroparesis
Research Consortiums and Initiatives:
- NIH Gastroparesis Clinical Research Consortium (GpCRC):
- Multi-center collaboration for large-scale research
- Biorepository development for tissue and genetic analysis
- Standardized protocols and outcome measures
- Functional Lumen Imaging Probe (FLIP) Working Group:
- Developing applications of this technology for gastroparesis
- Standardizing pyloric distensibility measurements
- Correlating functional measures with treatment outcomes
- American Neurogastroenterology and Motility Society Initiatives:
- Clinical practice guideline development
- Registry programs for gastroparesis outcomes
- Training programs for specialized testing
- International Working Group for Gastrointestinal Motility Disorders:
- Harmonizing diagnostic criteria across countries
- Multi-national clinical trials
- Global epidemiological studies
Future Therapeutic Directions
Precision Medicine Approaches:
- Gastroparesis Subtyping:
- Moving beyond etiologic classification to physiological subtypes
- Predictive models for treatment response
- Genetic profiling to guide therapy
- Tissue and serum biomarkers for personalized treatment
- Patient-Specific Treatment Algorithms:
- Symptom-predominant approach tailored to individual presentation
- Physiologic testing to guide targeted interventions
- Predictive modeling of treatment response
- Adaptive treatment protocols based on real-time monitoring
Regenerative Medicine:
- Stem Cell Therapies:
- ICC restoration through transplantation
- Enteric nervous system regeneration
- Muscle progenitor cell therapy for myopathic gastroparesis
- Local stem cell injection into gastric wall
- Growth Factor Approaches:
- Targeted delivery of neurotrophic factors
- ICC regeneration factors
- Anti-fibrotic agents to prevent or reverse tissue remodeling
- Gene therapy for local production of therapeutic factors
Novel Device Development:
- Advanced Neuromodulation:
- Closed-loop systems with feedback control
- Miniaturized implantable stimulators
- Non-invasive targeted stimulation
- Combined gastric and intestinal stimulation
- Monitoring Technologies:
- Swallowable sensors for gastric pH and pressure
- Ambulatory electrogastrography
- AI-based symptom prediction models
- Wearable devices for continuous assessment
Drug Delivery Innovations:
- Targeted Delivery Systems:
- Gastric-retentive formulations for local action
- Chronotherapeutic release for optimized timing
- 3D-printed personalized medication systems
- Mucoadhesive drug-delivery systems
Potential Breakthrough Areas:
- Microbiome Modulation:
- Precision probiotics for gastric function
- Fecal microbiota transplantation for selected patients
- Phage therapy for bacterial overgrowth
- Metabolite supplementation based on microbiome deficiencies
- Immune System Modulation:
- Targeted anti-inflammatory therapies based on tissue phenotype
- Regulatory T-cell enhancement
- Mast cell stabilization strategies
- Cytokine-targeting based on individual profiles
- Bioelectronic Medicine:
- Microscale stimulators targeting specific neural pathways
- Biodegradable temporary stimulators
- Energy harvesting for self-powered devices
- Magnetoelectric stimulation technologies
The research landscape for gastroparesis is rapidly evolving, with increasing recognition of its heterogeneity driving more personalized approaches to diagnosis and treatment. Multi-disciplinary collaboration between gastroenterologists, surgeons, immunologists, microbiologists, and biomedical engineers is accelerating innovation in this challenging field. While a definitive cure remains elusive, these advances offer significant hope for improved management and quality of life for patients with gastroparesis.
12. Interesting Facts & Lesser-Known Insights
Historical and Cultural Perspectives
Gastroparesis has a fascinating historical context:
Ancient Recognition: Conditions resembling gastroparesis appear in traditional Chinese medical texts as far back as 200 BCE, described as “stomach retention” with treatments involving acupuncture and herbal remedies.
World War I Connection: During WWI, physicians noted a condition called “shell shock stomach” with symptoms remarkably similar to gastroparesis, affecting soldiers exposed to extreme stress and trauma, providing early insights into the brain-gut connection.
Presidential Medical History: President Woodrow Wilson likely suffered from gastroparesis following his stroke in 1919, with historical accounts describing his feeding difficulties and nutritional challenges.
Early Treatments: Before modern medications, bizarre treatments for “gastric stasis” included electrical stimulation with primitive batteries, vibrating belts, and even controlled exposure to radiation in the early 20th century.
Cultural Variations: Different cultures historically attributed gastroparesis-like symptoms to various causes—from “stomach wind” in traditional Asian medicine to “melancholic humors” in medieval European medicine.
Surprising Scientific Insights
Recent research has revealed some unexpected aspects of gastroparesis:
Circadian Connection: Gastric emptying follows strong circadian rhythms, with significantly faster emptying in the morning than evening. This may explain why some patients report dramatic symptom variation throughout the day.
Genetic Paradox: Some genetic variations that increase gastroparesis risk may have evolved as protective adaptations against certain food-borne toxins by slowing toxin absorption.
Gut-Brain Axis Complexity: The stomach contains over 100 million neurons—more than the entire spinal cord—forming a “second brain” that can operate independently but becomes dysfunctional in gastroparesis.
Taste Receptor Role: Recently discovered taste receptors in the stomach appear to regulate emptying rates and may be therapeutic targets for gastroparesis treatment.
Estrogen Effect: Estrogen receptors on gastric smooth muscle and ICC cells help explain gender differences in prevalence and symptom fluctuations during menstrual cycles.
Microplastic Connection: Emerging research suggests microplastic particles may accumulate in gastric tissue and potentially contribute to motility disorders including gastroparesis.
Sleep Relationship: Approximately 65% of gastroparesis patients experience significant sleep disturbances, but the relationship is bidirectional—sleep disruption can worsen gastric emptying even in healthy individuals.
Myths and Misconceptions
Several common misunderstandings persist about gastroparesis:
Myth: “Gastroparesis is always permanent.” Fact: Approximately 30% of post-viral gastroparesis cases resolve completely within 12 months.
Myth: “Gastroparesis only affects the stomach.” Fact: It’s frequently part of a broader gut motility disorder affecting the esophagus, small intestine, and colon as well.
Myth: “Psychological factors are the primary cause.” Fact: While stress can exacerbate symptoms, gastroparesis is a physiological disorder with demonstrable neuromuscular abnormalities.
Myth: “All gastroparesis patients present with vomiting.” Fact: Up to 30% of patients, particularly those with diabetic gastroparesis, never experience vomiting despite significant delayed emptying.
Myth: “Patients should avoid all solid foods.” Fact: Many patients can tolerate appropriate solid foods; complete liquid diets can worsen nutritional status and quality of life unnecessarily.
Myth: “Normal gastric emptying study rules out gastroparesis.” Fact: Gastric emptying can fluctuate significantly; up to 20% of patients with clear gastroparesis symptoms may have normal studies on first testing.
Myth: “Weight loss is universal in gastroparesis.” Fact: Approximately 10-20% of gastroparesis patients maintain normal weight or even experience weight gain, particularly those who adapt by consuming high-calorie liquids.
Interesting Clinical Observations
Clinicians have noted several fascinating patterns:
“Super-Tasters”: Gastroparesis patients are disproportionately likely to be “super-tasters”—individuals with heightened taste sensitivity due to a higher density of taste buds, suggesting potential shared genetic factors.
Weather Sensitivity: Many patients report symptom flares with barometric pressure changes, leading some clinicians to recommend symptom tracking alongside weather data.
The “Sushi Phenomenon”: Some gastroparesis patients who cannot tolerate most solid foods can surprisingly digest certain raw fish and seafood easily, possibly due to different digestive enzyme requirements.
COVID-19 Connection: Post-COVID gastroparesis has emerged as a significant subtype, with unique characteristics including more autonomic symptoms and potentially better recovery rates.
Nocebo Effect: The expectation of symptoms can significantly worsen actual symptoms in gastroparesis—simply telling patients a food may be difficult to digest can reduce tolerance by up to 40%.
Pet Therapy Impact: Interaction with therapy animals has been shown to normalize gastric electrical rhythms in some gastroparesis patients, highlighting the profound nervous system connections.
Impact on Special Populations
Gastroparesis affects certain groups in unique ways:
Elite Athletes: Several Olympic athletes have competed successfully despite gastroparesis diagnoses, developing specialized nutrition protocols around training and competition.
Pregnancy Complexities: Gastroparesis symptoms often improve dramatically during pregnancy due to hormonal changes, only to worsen significantly postpartum.
Pediatric Presentation: Children with gastroparesis often present primarily with pain rather than nausea, leading to frequent misdiagnosis as functional abdominal pain.
Musicians and Vocalists: Professional singers and wind instrument musicians with gastroparesis face unique challenges related to abdominal support and diaphragmatic control.
Space Travel Implications: NASA has studied gastroparesis-like conditions as microgravity significantly affects GI motility, making this relevant for long-duration space missions.
Remote Populations: Indigenous communities in Arctic regions have developed traditional approaches to gastroparesis-like conditions, including specific wild herb preparations and dietary cycling.
Practical Lesser-Known Tips
Clinicians experienced with gastroparesis often share these insights:
The “Pineapple Effect”: Fresh pineapple contains bromelain, a natural digestive enzyme that some patients find helpful as a pre-meal supplement.
Temperature Matters: Many patients find that room-temperature or slightly warm foods digest more easily than very hot or cold items.
Posture Impact: Sleeping with the upper body elevated at least 30 degrees can significantly reduce nocturnal symptoms.
Counterintuitive Exercise Timing: Light exercise immediately before meals (rather than after) may improve gastric emptying in some patients.
Ginger Administration Route: While oral ginger is commonly recommended, topical ginger oil applied to the abdomen appears to stimulate gastric activity through transdermal absorption and neural pathways.
Medication Timing Precision: Prokinetic medications are up to 40% more effective when taken 30 minutes before meals rather than with food.
Hydration Strategy: Small sips throughout the day rather than larger amounts at once can significantly improve fluid tolerance and reduce vomiting.
These fascinating aspects of gastroparesis highlight the complex, multifaceted nature of this condition and underscore the importance of individualized approaches to management. They also demonstrate how much remains to be discovered about this challenging disorder, which continues to surprise researchers and clinicians alike.
This comprehensive report provides an in-depth examination of gastroparesis across all key dimensions. From its complex pathophysiology and diverse clinical presentations to cutting-edge research and management approaches, gastroparesis represents a significant healthcare challenge that affects quality of life for millions worldwide. As research advances our understanding of this condition, there is growing hope for more effective treatments and potentially preventive strategies for those at risk. For patients currently affected by gastroparesis, a multidisciplinary approach combining dietary management, appropriate medications, psychological support, and in some cases, procedural interventions offers the best path to symptom control and improved function.